Solid Electrolytes & Solid State Batteries
In this field, we work on ion-conducting garnets, perovskites and composites, design and synthesize novel solid electrolytes, and develop solid-state batteries with high safety, high energy density and long lifetime.
Our current works are:
1) Ionic transport in oxides with garnet and perovskite structures, and oxide-polymer composites:
(1) Ionic conduction in Li7La3Zr2O12 (LLZO) garnets, and the strategy to enhance the ionic conductivity by proper doping. By Ga and Rb co-doping, the ionic conductivity of LLZO can be enhanced to: = 1.62×10-3 S/cm, tion = 1.
= 1.62×10-3 S/cm, tion = 1.
(2) The surface property of LLZO garnets. Without any surface modification, Li2CO3-free LLZO shows an intrinsic “lithiophilicity” characteristic, leading to a continuous and tight Li/LLZO interface with an ultra-low interfacial resistance of 49 Ω cm2.
(3) Physical origin of the low grain-boundary conductivity of Li3xLa0.67-x□0.33-2xTiO3 (LLTO, 0.12 ≤ 3x ≤ 0.50, □ represents the A-site vacancy). The low G.B. conductivity in LLTO is due to the depletion of lithium ions in the G.B. space-charge layers.
(4) Ionic conduction in oxide-polymer composites. The Li+ conduction in LLZO-PEO composites takes place via the fast conduction in the LLZO/PEO interfacial regions and the percolation of the interfacial regions.
2) Novel solid electrolytes:
(1) New Li-ion conductor LiTaSiO5 was designed and synthesized, whose electrical properties are: = 3×10-5 S/cm, tion = 1.
= 3×10-5 S/cm, tion = 1.
(2) New solid electrolyte was derived from Zr-based MOFs, whose electrical properties are: = 3.2×10-4 S/cm, tion = 0.33.
= 3.2×10-4 S/cm, tion = 0.33.
3) Solid-state batteries:
(1) In-situ polymerization combines the advantages of both liquid and solid electrolytes, while eliminates the disadvantages; representative electrical properties of in-situ polymerized electrolytes are: = 4.3×10-3 S/cm, tion = 0.55. Polymerized electrolytes are flexible and flame-retardant (Figure 3).
= 4.3×10-3 S/cm, tion = 0.55. Polymerized electrolytes are flexible and flame-retardant (Figure 3).
Our current works are:
1) Ionic transport in oxides with garnet and perovskite structures, and oxide-polymer composites:
(1) Ionic conduction in Li7La3Zr2O12 (LLZO) garnets, and the strategy to enhance the ionic conductivity by proper doping. By Ga and Rb co-doping, the ionic conductivity of LLZO can be enhanced to:
(2) The surface property of LLZO garnets. Without any surface modification, Li2CO3-free LLZO shows an intrinsic “lithiophilicity” characteristic, leading to a continuous and tight Li/LLZO interface with an ultra-low interfacial resistance of 49 Ω cm2.
(3) Physical origin of the low grain-boundary conductivity of Li3xLa0.67-x□0.33-2xTiO3 (LLTO, 0.12 ≤ 3x ≤ 0.50, □ represents the A-site vacancy). The low G.B. conductivity in LLTO is due to the depletion of lithium ions in the G.B. space-charge layers.
(4) Ionic conduction in oxide-polymer composites. The Li+ conduction in LLZO-PEO composites takes place via the fast conduction in the LLZO/PEO interfacial regions and the percolation of the interfacial regions.
2) Novel solid electrolytes:
(1) New Li-ion conductor LiTaSiO5 was designed and synthesized, whose electrical properties are:
(2) New solid electrolyte was derived from Zr-based MOFs, whose electrical properties are:
3) Solid-state batteries:
(1) In-situ polymerization combines the advantages of both liquid and solid electrolytes, while eliminates the disadvantages; representative electrical properties of in-situ polymerized electrolytes are:

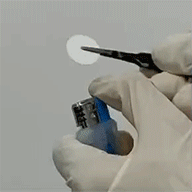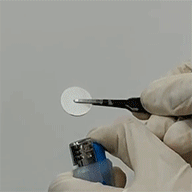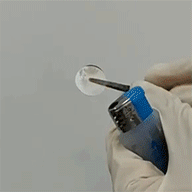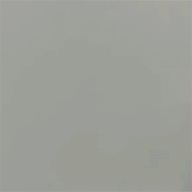
Figure 3. Flexible and flame-retardant polymer electrolytes.
(2) Solid–state batteries: Our LiFePO4||Li battery sustains a retention over 94% over 1500 cycles at 2 C (Figure 4), and the NCM622||Li battery achieves 81% retention after 400 cycles at 2 C.
Figure 4. Cycling stability of the LiFePO4||Li battery for 1500 cycles at a rate of 2 C and room temperature.
4) Electro-chemo-mechanical actuators:
The volume change involved in the charging/discharging process of solid-state batteries is normally believed to be detrimental, however, we are now developing electro-chemo-mechanical actuators by taking the advantage of the volume change, in collaboration with Weizmann Institute of Science, Israel.
